Crimean-Congo hemorrhagic fever (CCHF) is a virulent human disease and the most wide spread tick-borne viral infection of human with a case fatality rate ranging from 5 to 50%. This is the widest geographic range virus of all tick-borne viruses
The Crimean-Congo hemorrhagic fever (CCHF) virus has the widest geographic range of all tick-borne viruses and is endemic in more than 30 countries across four regions: Africa (democratic republic of Congo , Uganda , Nigeria , south Africa , Senegal and Sudan) Asia (China , Pakistan , India , Afghanistan Uzbekistan , Tajikistan , Kazakhstan ) Europe Mediterranean region (Russia , Turkey , Bulgaria , Kosovo , Greece , Spain) Middle east ( Iran , Iraq , Oman, Saudi Arabia , Kuwait , United Arab Emirates (UAE ) and most recently the Iberian Peninsula.
CCHF is also known as Congo fever, Central Asian hemorrhagic fever, Congo virus disease, Hungribta (blood taking), Crimean hemorrhagic fever, Khunymuny (nose bleeding), Karakhalak (Black Death), and viral tick-borne hemorrhagic fever disease.
Etiology:
The etiological agent, CCHF virus (CCHFV or congo fever virus), is a single-stranded, negative sense RNA virus classified within the Nairovirus genus of the family Bunyaviridae.
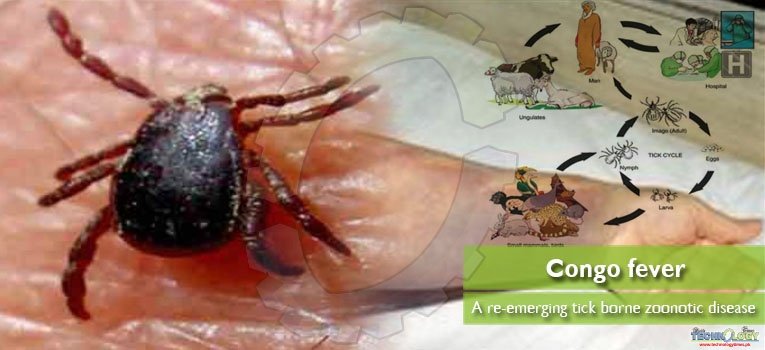
It is maintained in an enzootic cycle involving tick-mediated transmission between several species of vertebrate including wild and domestic mammals. This has been isolated from 30 species of family Ixodidae especially those of the genus Hyalomma, both a reservoir and a vector for the virus. It is possibly because both juvenile and adult forms vigorously look for hosts for the blood meals required at each stage of maturation.
Congo virus sustained in common mammals such as cattle, goats, sheep, small mammals, rodents, and birds. In these mammals the infection is mainly asymptomatic, serve as amplifying hosts for the virus.
It is considered an emerging arboviral zoonotic disease in many countries, possibly due to increased vector bionomics and climate change
Incubation period
The length of the incubation period varies depending on several factors, including the viral dose and the route of exposure, and is often shorter following nosocomial infection. Following infection by a tick bite, the incubation period is usually one to three days, with a maximum of nine days. The incubation period following contact with infected blood or tissues is usually five to six days, with a documented maximum of 13 days.
History
The disease was first described in the Crimea in 1944 and given the name Crimean hemorrhagic fever. In 1969 it was recognized that the pathogen causing Crimean hemorrhagic fever was the same as that responsible for an illness identified in 1956 in the Congo, and linkage of the two place names resulted in the current name for the disease and the virus.
Peoples at risk
The disease occurs most frequently among occupational groups including people involved in the livestock industry. This include agricultural workers, slaughterhouse workers, Health care workers, medical personnel and veterinarians, who are also a vulnerable group to the infection.
In addition, person-to-person transmission can be occurs due to direct or indirect contact with the skin, mucous membranes, or body fluids of infected patients. Nomads travelling place to place along with their herds of animals are said to be responsible for spread of disease, both to animals and animal handlers.
Epidemiology
In Pakistan, the first Congo fever (CCHF) case was reported in 1976; an additional 14 outbreaks were reported during 1976 – 2010 period and since then there has been a biannual surge of Congo virus cases in the Country.
Pakistan is an endemic country and has the fourth highest number of cases of Congo fever infection in Asia, after Turkey, Russia, and Iran. Cases usually appear between March and May and again between August and October. Several outbreaks of the disease have been reported in the country, spread over a wide geographic area.
Balochistan, Karachi, and Rawalpindi are the most affected regions. However, the situation has deteriorated in Quetta city, where, since January, 2016, 84 patients have been suspected of being infected with Congo fever at the Fatima Jinnah General and Chest Hospital (FJCH). Of these 84 patients in the provincial capital, 22 were confirmed as having the disease and ten died.
The incidence of Congo fever peaks in June and October but cases occur throughout the year. Crimean Congo Haemorrhagic Fever (CCHF) cases are reported continuously from epidemiological week 8, 2014.
So far total 100 suspected cases, 38 laboratory confirmed, and 28 deaths (of these 14 laboratory confirmed) have been reported. Most of the cases are from Balochistan province 46 suspected (13 cases belongs to Afghanistan), 10 Laboratory confirmed and 9 deaths. 30 suspected, 17 Laboratory confirmed (12 from Afghanistan) and 10 deaths (7 Lab confirmed) were reported from Khyber Pakhtunkhwa province. 4 suspected cases (2 from Afghanistan), 3 Laboratory confirmed and 2 deaths were reported from Islamabad. 15 suspected CCHF cases (7 Laboratory confirmed), 6 deaths were reported from Punjab province. 2 laboratory confirmed cases (1 death) were reported from Sindh province.
The disease is reportedly endemic in Afghanistan, Iran, and Pakistan, particularly in the areas bordering these countries where frequent movement of nomads with their animals is concentrated. Trade in animals and animal skins within Pakistan, and between Pakistan, Iran, and Afghanistan, is thought to play a major role in the spread.
The Congo fever virus infected people are among who handle animals or their skins, slaughter infected animals, and/or come into close contact with ticks or Congo fever patients. Since ticks are more likely to be or to have recently been feeding on these animals. This may lead to an increase in the number of Congo fever virus infections.
This occurs due to careless practices during the slaughtering of animals, inadequate knowledge of the disease, and the dissemination of Congo fever virus through uncontrolled animal movements in and between countries. However, the risk of infection is generally of less concern, gloves, aprons, goggles etc are not commonly used. This is a key factor in the zoonotic transmission of Congo fever virus from viremia animals during slaughter and butchering.
Eid-al-Adha and Congo virus
Zoonotic transmission as a result of the slaughter and butchering of domestic animals increases during preparations for Eid-al-Adha. An annual religious festival celebrated by Muslims. During Eid-al-Adha many animals, including goats, sheep, cows, and camels are slaughtered.
It was hypothesized that the festival could play an important part as people could come into contact with domestic or imported animals potentially infected with Congo fever virus. The National Institute of Health, Pakistan showed there was no correlation, and that Congo fever cases have coincided with the peak tick proliferation during the preceding 8–10 years.
As the timing of this festival follows the Islamic lunar calendar, it moves approximately 10 days earlier each year. Significantly in the next 10–20 years Eid-al-Adha will occur in the summer and spring months, when sacrificial animals are more likely to be viremic for Congo fever virus.
As a result of the transmission of the virus from infected blood-feeding ticks which are more active during these times. Animals are housed in open spaces and private houses until they are slaughtered during the 3 days of Eid-ul-Adha. This allows the Congo fever virus, which is carried by a tick that inhabits the animal hide, to be transmitted through unprotected contact with live animals.
This also occurs through contact with animal blood subsequent to its slaughter. There is concern therefore, that the slaughter of viremic animals during these periods may facilitate the spread of Congo fever virus to humans which may cause secondary and tertiary human-to-human spread and subsequent outbreaks.
Modelling of climate change has demonstrated that a rise in temperature and a decrease in rainfall in the Mediterranean region could result in a sharp rise in the distribution of suitable habitats for Hyalomma ticks thus increasing the pool of human populations at risk.
Clinical sign and symptoms
Congo fever virus is characterized by a sudden onset of symptoms with high fever, chills, myalgia, sore eyes, severe headache, photophobia (sensitivity to light), dizziness, back, and abdominal pains. Additional symptoms can include nausea, vomiting, diarrhea, neuropsychiatric, and cardiovascular changes. Fever is often very high (39–41°C) and can be constantly elevated for 5–12 days or may be biphasic.
Other clinical signs include tachycardia (fast heart rate), lymphadenopathy (enlarged lymph nodes), and a petechial rash (a rash caused by bleeding into the skin) on internal mucosal surfaces, such as in the mouth and throat, and on the skin and Vagina. Large ecchymosis and uncontrolled bleeding from venipuncture sites are common features. The convalescent period begins in survivors about 10–20 days after the onset of illness.
Problems regarding control
- Poor recognition of Congo fever by physicians
- Single sporadic cases tend to occur in rural areas, and many patients develop a mild, non-specific illness, without a recognizable hemorrhagic fever syndrome.
- A lack of identified national Congo fever prevention, control programs and poor reporting practices may result in under-reporting.
- It is difficult to prevent or control Congo fever virus infection in animals and ticks, since the tick–animal–tick cycle continues unnoticed and viral infection in animals is usually not apparent. Additionally, tick vectors are numerous and widespread, making tick control with arachnicide realistic only for well-managed livestock production facilities, which are uncommon in the region.
- The main alternative to chemical tick control is tick immunity through animal vaccination. Second, in the absence of a human Congo fever vaccine, the only way to reduce infection in humans is by raising awareness of risk factors and educating people about the preventive measures necessary to reduce exposure to the virus. However, as the populations at risk of Congo fever are nomads, farmers, and animal herders living in remote and disadvantaged areas, considerable effort and innovative approaches will be required to reach them with information, education, communication, and social mobilization activities.
- Controlling infection in the health care setting requires strict adherence to standard infection control measures. Including basic hand hygiene, use of personal protective equipment, safe injection practices, and safe burial practices. The risk of nosocomial infection in health-care workers is well documented and can be extremely high, especially during the hemorrhagic period of disease.
Control Measures
The best means of preventing disease is to avoid or minimize exposure to the virus. Persons in high-risk occupations (i.e. slaughterhouse workers, veterinarians, sheep herders, etc.) should take every precaution to avoid exposure to virus infected ticks or virus-contaminated animal blood or other tissues. For example, by using tick repellents and by wearing gloves and limiting exposure of naked skin to fresh blood and other tissues of animals are effective practical control measures.
Treatment
The treatment of confirmed patients is mainly supportive (painkillers, antiemetic for vomiting, anxiolytic for agitation, +/-antibiotics and/or antimalarial drugs). Generally this includes the basic management of symptoms with intravenous fluids, electrolyte balance, renal function, blood pressure, oxygenation, careful rehydration and blood products (platelets, fresh frozen plasma).
There is evidence that early diagnosis and prompt management of the symptoms of cases with supportive treatment results in a better clinical outcome.
Ribavirin has been used by a number of countries for treatment. When administered within the 1–2-day window, ribavirin was found to be effective in reducing the case fatality rate of mild cases. Consequently, there is currently no antiviral treatment for Congo fever approved by the U.S. Food and Drug Administration (FDA).
Discussion
Crimean-Congo hemorrhagic fever (CCHF or Congo fever) is a serious public health problem that requires efforts from all stakeholders to control it from further spreading through maintaining high index of suspicion, early diagnosis and initiation of treatment along with a strong focus on prevention.
Congo fever is a clear and growing health threat in the WHO EMR (Eastern Mediterranean). Some new areas are reporting cases, showing a geographic extension of the disease that is probably linked to the trade in livestock and the spread of infected ticks by migratory birds.
According to ecological models, the rise in temperature and decrease in rainfall in the WHO EMR could result in a sharp rise in the distribution of suitable habitats for Hyalomma ticks and subsequently drive Congo fever virus infection northwards. Thus, the development and implementation of a strategic framework for the prevention and control of Congo fever is important to curb the new threats posed by Congo fever virus.
This article is jointly authored by Dr. Shah Zain Ali1*, Dr. Muhammad Ashraf1, Muhammad Shafi Hasni2, Amir Munir2, Hassan Nawaz1. The authors are from 1. Institute of Microbiology, University of Agriculture Faisalabad-Pakistan, 2. Department of Parasitology, University of Agriculture Faisalabad-Pakistan. The Corresponding author can be reached at shahzainali3519@gmail.com