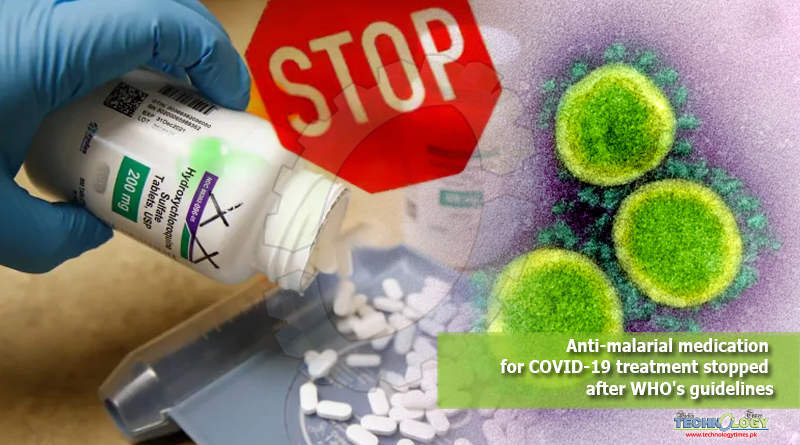
The Pakistani health specialists have chosen to incidentally suspend the clinical trials of patients to Anti malarial medication for COVID 19 patients after the WHO choice to briefly end the HCQ concentrate over security concerns.
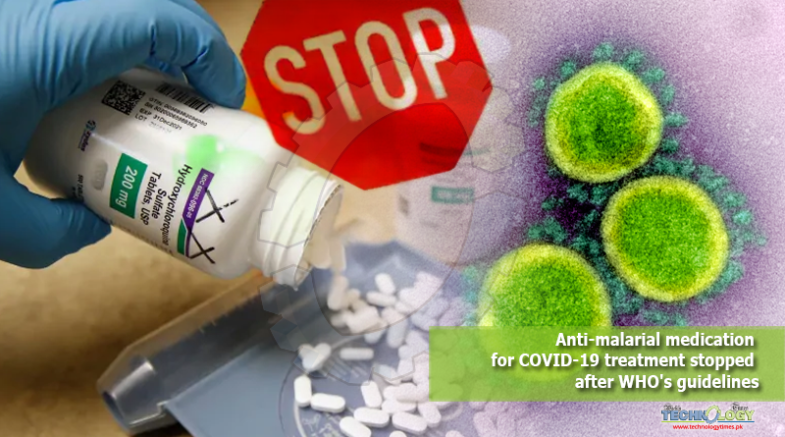
The Pakistani health specialists have chosen to incidentally suspend the clinical trials of patients to anti malarial medication for COVID 19. Since malarial medication chloroquine and its simple hydroxychloroquine (HCQ) for the treatment of COVID-19 patients after the World Health Organization (WHO) choice to briefly end the HCQ concentrate over security concerns.
“The Drug Regulatory Authority of Pakistan [Drap] will issue notices to the health facilities carrying the anti-malarial trials in Pakistan to put them on hold till further instructions,” Special Assistant to Prime Minister Dr Zafar Mirza told, saying that the Executive Group of the Solidarity Trial, representing 10 countries had decided to review and temporarily suspend the clinical trials of HCQ in COVID-19 patients.
Well before the WHO’s advisory, several hospitals treating the COVID-19 patients in Karachi and other cities of the country had halted the use of chloroquine and HCQ for the treatment of patients infected with COVID-19, saying that the drug was not producing desired results when used alone or in combination with anti-biotic azythromycin.
“We had already reduced the use of chloroquine and hydroxychloroquine for the treatment of COVID-19 patients as data indicated that it was not proving very much effective. As far as the trials are concerned, the WHO has paused the trials to reassess the safety data so we paused it too,” said Dr. Faisal Mahmood, an infectious diseases expert at the Aga Khan University Hospital in Karachi.
More than 400 medical clinics in 35 nations, including Pakistan, were effectively selecting patients and almost 3,500 patients had just been enlisted from 17 nations, the WHO stated, including that it started the Solidarity Preliminary two months back to assess the health and adequacy of four medications and medication mixes against COVID-19.
The health experts in Pakistan said that right now, the University of Health Sciences (UHS) in a joint effort with a nearby pharmaceutical organization, Getz Pharma, was leading the clinical preliminaries of HCQ for the treatment of COVID-19 patients at two or three health offices in Lahore while some health offices in Karachi were additionally utilizing the counter malarial medications for the treatment of patients with coronavirus disease.
They said that last Friday, a presumed clinical diary, The Lancet, distributed an observational investigation on HCQ and chloroquine and its consequences for COVID-19 patients that had been hospitalized. “Creators of the examination revealed that among patients accepting the medication, when utilized alone or with a macrolide, they assessed a higher death rate,” said an authority of the national health administrations service of Pakistan.
The health official kept up that albeit both HCQ and chloroquine phosphate were being utilized tentatively at different health unites in Pakistan for the treatment of COVID-19 patients, clinical trials still couldn’t seem to begin in the nation as clinical colleges and offices were currently enrollment. “Every single new trial should experience an audit dependent on the present proof. However, recall that multiple occasions, a various number of trials are expected to thoroughly know the appropriate response,” the authority included.
To a question, he said all the trials had an oversight instrument to check whether any between time aftereffects of the preliminary or different preliminaries on the planet are there and besides, if any progressions or relinquishment is required.
Citing the WHO, authorities in the Service of National Health Institute said the Official Gathering of the Solidarity Preliminary, speaking to 10 of the taking an interest nations, met keep going Saturday and concurred on leading an extensive investigation and basic evaluation of all the proof accessible all around.
The survey will consider information gathered so far in the Solidarity Preliminary and specifically the accessible vigorous randomized information, to sufficiently assess the potential advantages and damages of the medications.