Nitinol an emerging Shape memory material employed in basic cardiovascular stents to high-level aerospace applications by NASA

By Muhammad Mubeen ¹ & Arsalan Ahmad ²
- Introduction:
Shape Memory Alloys (SMA) or metallic (memory) materials as the name implies are famous because they remember their shape, due to thermos-elastic and mechanical (martensitic) phase transformations on an atomic level. These alloys have superiority in terms of large recoverable strain and exert continuous force during applications.
- Historical Background:
The first shape memory alloy was discovered by Arne Olander in the year 1932, and later Nitinol was prepared by Buheler and Wiley at the Naval Ordnance Laboratory (suffix of “nol” in the alloy name) in 1960. After discovery until a decade, commercialization of Nitinol alloy was not possible due difficulty of melting, processing, and fabrication of the material.
- Nitinol:
Nickel-titanium alloy named “Nitinol” is a remarkably advanced shape memory material, belongs to the class of intermetallic equiatomic binary alloys of NiTi group as demonstrated in Figure.1. It is a family of titanium-based alloys that contain a nearly equal amount of nickel and titanium (50-50%). It has great potential for use in automotive, aerospace, biomedical and even civil engineering applications. It has proven to be the most advantageous alloy among all known shape memory alloys, attracting the global scientific community in recent decades.
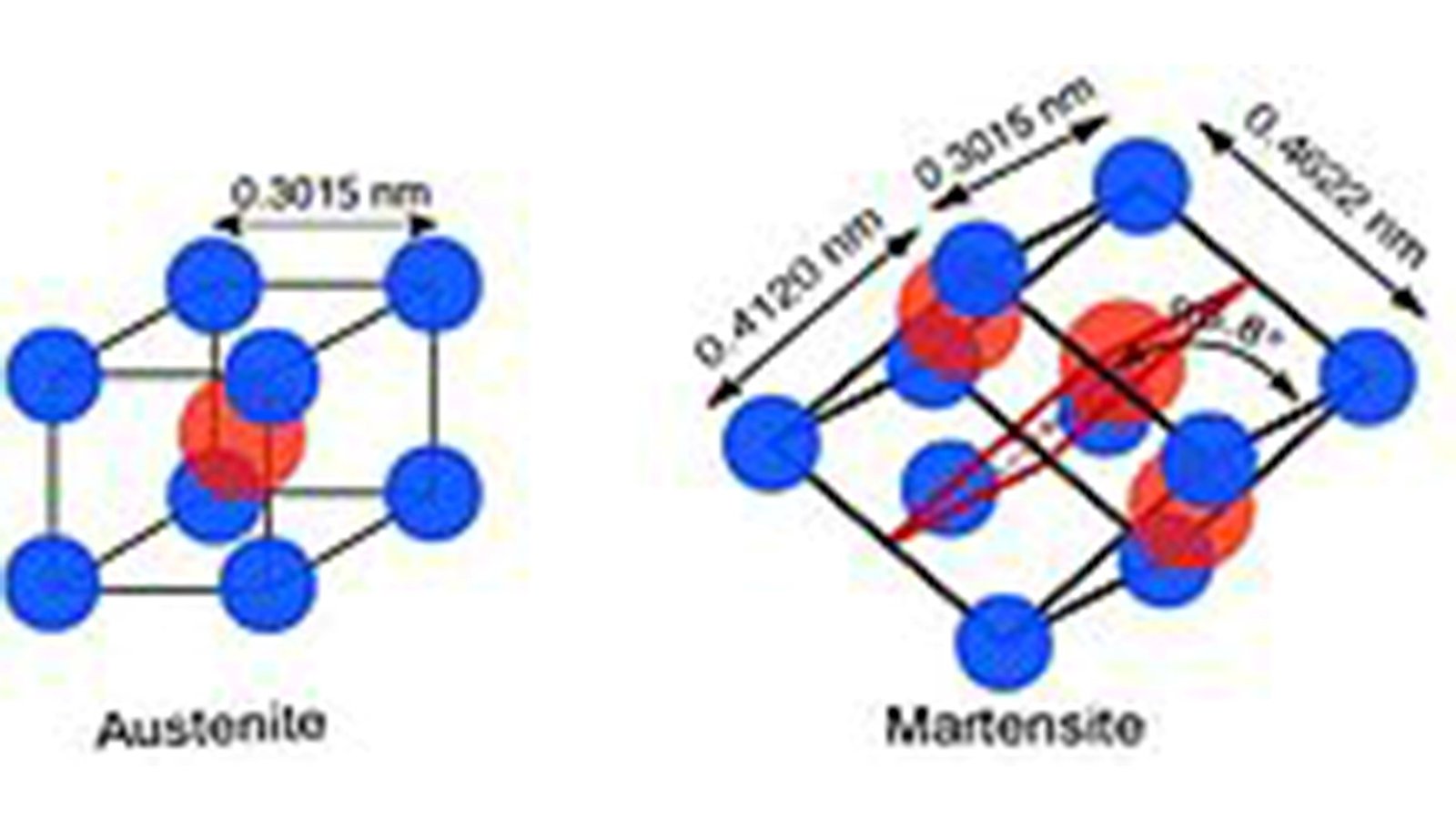
Figure.1: Temperature-based different unit geometries of Nitinol
- Commercial Availability of Nitinol:
As shown in Figure.2, Nitinol components are available in various forms including wires, tubes, sheets, foils and springs in various standards either in machined or processed conditions.

Figure.2: Basic Nitinol-based Components
- Phase Diagrams and Metallographic Features:
The Nitinol phase diagram is partialized from the nickel-titanium alloy system as depicted in Figure.3. The B2 phase region is known to be very narrow at a temperature below 923K (649.85oC). It is generally accepted that the B2 phase region is only between 50 and 50.5 atomic percentage of nickel. If nickel content is higher than 50.5 atomic percent, then the alloy will decompose during cooling below 973K (699.85oC) to other products i.e. TiNi and TiNi3. While Ti3Ni4 and Ti2Ni3 are intermediate phases formed during transformations. In the central part of the phase diagram, where TiNi transforms to monoclinic (B19) martensitic phase and an important alloy formed in this regime is called by the specific name of “Nitinol”. For the titanium-rich side, the equilibrium phase is Ti2Ni and TiNi. The metallographic structures of NiTi alloy (50:50) as shown in Figure.3, represents “dendrites” observed under magnification of 200x at room temperature.

Figure.3: Partial phase diagram (Left) of Nickel-titanium from 45% Ni to 59% Ni (Upper Right) & (Lower Right) Microscopic illustrations of phases.
- Properties:
The most unique properties of Nitinol are following:
- Shape Memory Effect (SME)
- Superelasticity
- Shape Memory Effect (SME):
The shape memory property allows this metal to “remember” its original shape and can recover it when heated above its transformation temperature. It happens due to the dissimilarities in crystal structures of nickel and titanium Figure. 4 depicts the mechanism. In this case, the parent austenite phase is stable above austenite finish temperature (Af) and when it’s cool to a temperature below the martensite finish temperature (𝑀f), it transforms to diffusionless twinned oriented martensitic phase. For the shape memory effect, the material is in a martensitic state at test temperature, in general. When an external force is applied, martensite changes to detwinned martensite. Upon removal of the force, the material remains in its detwinned martensitic state. When we heat this material above the austenite finish temperature (𝐴f), reverse transformation occurs from detwinned or deformation-induced martensite to parent phase and the original shape is recovered back. This all mechanism is named as the shape memory effect (SME). In the case of SME, heating above the austenite transformation temperatures is a must to recover the original shape.
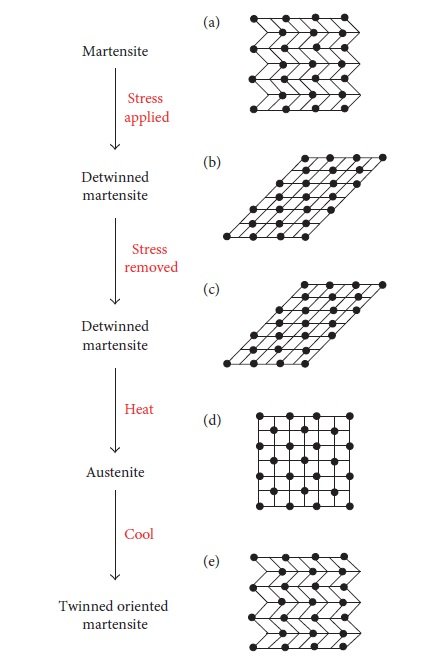
Figure. 4: Mechanism of SME at a temperature below 𝑀𝑓. (a) Martensite at the test temperature. (b) Detwinned martensite upon application of stress. (c) Detwinned martensite upon removal of stress. (d) Austenite upon heating above 𝐴𝑓. (e) Martensite upon cooling below 𝑀𝑓 (test temperature).
- Superelasticity:
The term Superelasticity is sometimes also known as “pseudoelasticity”. Nitinol depicts extraordinary elasticity which is approx. 11 to 33 times more than that of an ordinary metal. In this case, the test temperatures are well above the austenitic finish temperature or in between the austenite start (𝐴s) and austenite finish (𝐴f) temperatures and the material is examined in an austenitic state at the test temperature. When force is applied, this austenite transforms to stress-induced martensite. However, this type of martensite is stable only under the application of stress, and when the stress is removed, the material reverts to austenite, as represented in Figure.5. In the case of superelasticity, heating is not required to recover the original shape as martensite in this case is stable only under the application of stress.
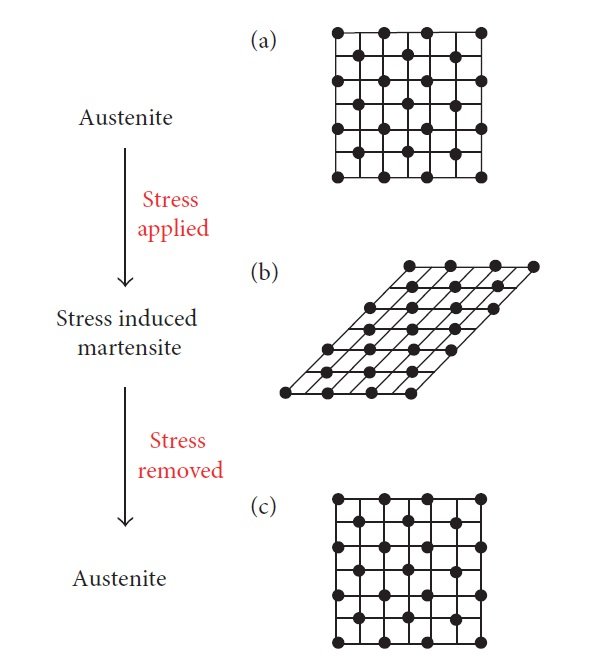
Figure. 5: Mechanism of superelasticity at a temperature above 𝐴𝑓. (a) Austenite at the test temperature. (b) Stress-induced martensite upon application of stress. (c) Austenite upon removal of stress.
- Manufacturing and Fabrication Techniques:
Several methods can be used in the manufacture and fabrication of Nitinol or nickel-titanium alloys.
- Manufacture:
- Vacuum Melting Methods
- Hot Working
- Cold Working
- Vacuum Melting Methods:
Nitinol ingots are melted by using one of the vacuum melting methods or a combination like:
- Vacuum Induction melting (VIM)
- Vacuum arc remelting/melting (VAR/VAM)
- Electron beam melting (EBM)
- Plasma Arc Melting (PAM)
In general, due to the high reactivity of titanium, vacuum melting methods are preferred. However, inert atmospheres can also be utilized effectively.
- Hot Working:
Nitinol ingots are generally hot worked in the temperature range of 700oC to 900oC as the successive stage in the process of wire, sheet or bar formation. For this purpose, different processes can be employed like:
- Extrusion
- Press forging
- Bar Rolling
- Rotary Forging
(Although, alloys do not react readily with air, cannot be hot worked.)
- Cold Working:
The most common method for cold working of Nitinol alloy is wire drawing that yields diameters as small as 0.05mm with superior surfaces. Although, work hardening happens instantly and constant heat treatment (annealing) is often needed. Proper heat treatment can reduce the deformation or residual stresses.
- Fabrication:
- Laser Cutting
- Electrical Discharge Machining (EDM)
- Welding
- Joining
- Machining
- Coating and Plating
- Applications of Nitinol:
There are lots of applications in which Nitinol material can be used. Some of the most useable are mentioned below:
- Biomedical Applications
- Cardiovascular coronary stents
- Orthodontic arc wires
- Guided wire in Endoscopes
- Bone fixation
- Artificial organs i.e. kidney, heat pump
- Industrial Applications
- Model heat engines
- Temperature controlling devices
- Mechanical watch spring
- Nitinol Sheets for punching, stamping and deep drawing
- Other Applications
- Eyeglass frames
- Self-bending spoons
- Insert for golf clubs
- Advantages & Limitations:
- Advantages:
- Bio-compatibility is superior
- The diverse field of application
- Good mechanical properties
- Limitations:
- Too Expensive
- Difficult processing and fabrication
- Poor fatigue properties
- Conclusions:
For over twenty to thirty years, Nitinol has been used in a wide range of medical as well as industrial devices and aerospace types of equipment. This article has mainly focused on today’s and future needs of this advanced emerging material. As the use of this magic metal becomes more widespread, the industry is facing many challenges regarding advanced applications that test the capability and superiority of the material. The chemical composition of Nitinol should carefully be controlled during processing (melting), it’s a key to improve fatigue life. The unique properties of the material make it suitable for various superior applications scripted above and make it a desirable choice for product designers/ material engineers whose design ambitions would probably be limited without the versatility of such kind of materials.
Muhammad Mubeen ¹ & Arsalan Ahmad ²
¹ ² Institute of Advanced Materials Bahauddin Zakariya University, Bosan road 60800 Multan, Pakistan