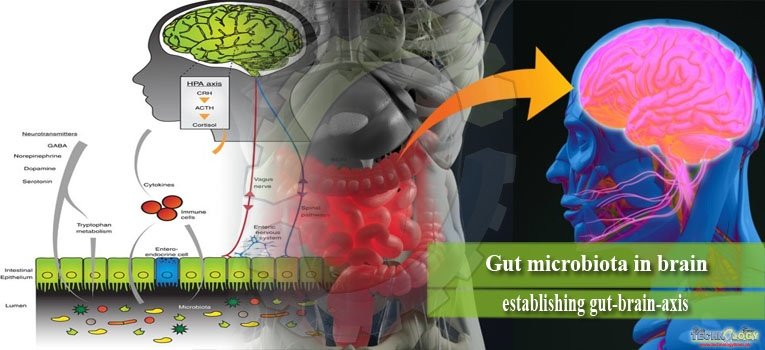
There is increasing interest in bidirectional signaling between the intestine and brain and their impact on mental health. Gut microbiota influences these signaling pathways, leading to the concept of a microbiota–gut–brain axis.
Microorganisms inhabit various sites of the human body, including the skin, nose, mouth and the gut. In particular the human gut is home to an enormous number of microorganisms, approximately 100 trillion bacteria cells, outnumbering human cells by an estimated 10 fold.

The microorganisms present in the gut are mainly bacteria and belong to more than 1,000 species, 90% of which belong to Firmicutes and Bacteroidetes. Each person has a distinct and highly variable composition of gut microbes. The composition of the gut microorganisms is called the gut ‘microbiota’, whereas the totality of the genes of the microbiota is called the ‘microbiome’.
The intestinal microbiota consists of a community of bacteria that colonize the gastrointestinal tract after birth and persist throughout adult life. It also include ‘transient’ bacteria, such as probiotic bacteria, which temporarily enters during ingestion of certain foods. The composition of the intestinal microbiota establishes during the first few years of life. Multiple factors shape it like maternal vertical transmission, genetic makeup of the individual, diet, medications such as antibiotics, gastrointestinal infections and stress.
Gut microbiota and brain
The ability of gut microbiota to communicate with the brain and thus modulate behavior is emerging as an exciting concept in health and disease. Bidirectional signaling between the gastrointestinal tract and the brain is regulated at neural, hormonal, and immunological levels. This construct is known as the brain–gut axis and is vital for maintaining homeostasis. Bacterial colonization of the intestine plays a major role in the post-natal development and maturation of the immune and endocrine systems. These processes are key factors underpinning central nervous system (CNS) signaling.
Recent research have seen a tremendous improvement in our understanding of the scale, diversity, and importance of the gut microbiome. This has been reflected in the form of a revised nomenclature to the more inclusive brain–gut–enteric microbiota axis and a sustained research effort to establish how communication along this axis contributes to both normal and pathological conditions.
Gut-Brain Axis
The gut-brain axis is a multi-component conceptual model describing the bidirectional communication pathways connecting cognitive and emotional centers in the brain with neuro-endocrine centers, the enteric nervous system (ENS) and the immune system. The ENS is a distinctive component of the autonomic nervous system (ANS) containing over 100 million of neurons. It is involved in the control of merely all gut functions including motility, secretion, absorption, and blood flow.
The ENS is present at the interface between the gut and brain. It is extremely receptive of chemical information arising from intestinal epithelium as well as the enteric endocrine and immune systems. It provides input to sensory pathways that signal to brain areas involved in emotion and cognition. On the other hand the ENS receives efferent information from the brain through autonomic neural connections (sympathetic and parasympathetic) and hormonal pathways, which in turn modulate digestive functions.

Mechanism of Action of Microbiota on Brain
The bidirectional communication between gut microbes and the brain occurs via a number of routes. They include the immune (cytokines) and neural (vagus and enteric nervous system) pathways.
The vagal pathway
The vagus nerve (cranial nerve X) plays a fundamental role in enabling signals from brain to gut and vice versa. Activation of the vagus nerve has also shown to have a marked anti-inflammatory capacity.
The immune pathway
The immune system provides a further route of communication between gut microbes and the brain. Microbiota and probiotic agents have direct effects on the immune system. The immune system also exerts a bidirectional communication with the CNS, making it a prime target for transducing the effects of bacteria on the CNS.
Production of neurotransmitters
Bacteria also have the capacity to generate many neurotransmitters and neuromodulators. For example, certain Lactobacillus and Bifidobacterium species produce gamma-aminobutyric acid (GABA); Escherichia, Bacillus and Saccharomyces spp.
Produce norepinephrine (NE); Candida, Streptococcus, Escherichia and Enterococcus spp. produce 5HT; Bacillus produces dopamine (DA); and Lactobacillus produces acetylcholine.
It is conceivable that secreted neurotransmitters of microorganisms in the intestinal lumen may induce epithelial cells to release molecules that in turn modulate neural signaling within the ENS, or act directly on primary afferent axons.

Effect of gut microbiota on brain functioning
The microbiota-gut–brain axis is a bidirectional system. Signals from the brain can influence motor, sensory, and secretory modalities of the gastrointestinal tract. In return, visceral messages from the gastrointestinal tract can influence brain function. The brain can indirectly affect enteric microbiota by effecting changes in gastrointestinal motility, secretion and intestinal permeability. In the other direction, enteric microbiota communicates with the brain via multiple mechanisms.
This gut brain signaling can alter brain morphology, and neurochemistry such as serotonin levels. The microbiome poises the peripheral immune homeostasis and predisposes host susceptibility to CNS autoimmune diseases such as multiple sclerosis. Neural, endocrine and metabolic mechanisms are also critical mediators of the microbiome–CNS signaling, which are more involved in neuro-psychiatric disorders such as autism, depression, anxiety, stress.

Treatment of problems caused by gut microbes
Increased interest in the effects of the intestinal microbiota on human health has resulted in attempts to optimize the composition of the microbiota by dietary interventions or with probiotics, prebiotics or both. The effects of probiotics depend on the properties of the microorganism used with both species- and strain-specific effects. Probiotics are mainly lactic acid bacteria (Lactobacilli and Bifidobacteria). It also include other bacterial species (Enterococci or some strains of E. coli) and yeast .
Probiotic bacteria are often present in foods such as yogurts and cheese, in food supplements, or as drugs. Prebiotics are compounds that support the proliferation of beneficial bacteria (Lactobacilli and Bifidobacteria) in the intestine and include some saccharides (e.g., inulin).
Probiotics have been shown to favorably influence the development and stability of the microbiota, inhibit the colonization by pathogens, influence the mucosal barrier by trophic effects on the intestinal epithelium, protect against physiological stress, and stimulate both specific and non-specific components of the immune system.
Conclusion
Microbes are essential part of our body. The human microbiota contributes in a number of important functions in the body. However there is a strong correlation between the interaction of microbes and the brain. This bidirectional signaling pathway is important for the survival of microbes as well as human beings. With the advancement in research, it has been found that these gut microbes have an effect on the functioning of brain. This relation however could be positive or negative. The role of microbiota in regulation of mood and stress is a field of interest for the researchers.